What Are Lithium-Ion Batteries?
Editor’s note: At a time when potentially risky energy storage technologies can be found in everything from consumer products to transportation and grid storage, UL Research Institutes helps to lay the groundwork for energy storage designs that are safe and reliable. As part of our work in this field, we want to share information on the foundations and current landscape of electrochemical safety.
What is a lithium-ion battery?
Lithium-ion is the most popular rechargeable battery chemistry used today. Lithium-ion batteries power the devices we use every day, like our mobile phones and electric vehicles.
Lithium-ion batteries consist of single or multiple lithium-ion cells, along with a protective circuit board. They are referred to as batteries once the cell, or cells, are installed inside a device with the protective circuit board.
What are the components of a lithium-ion cell?
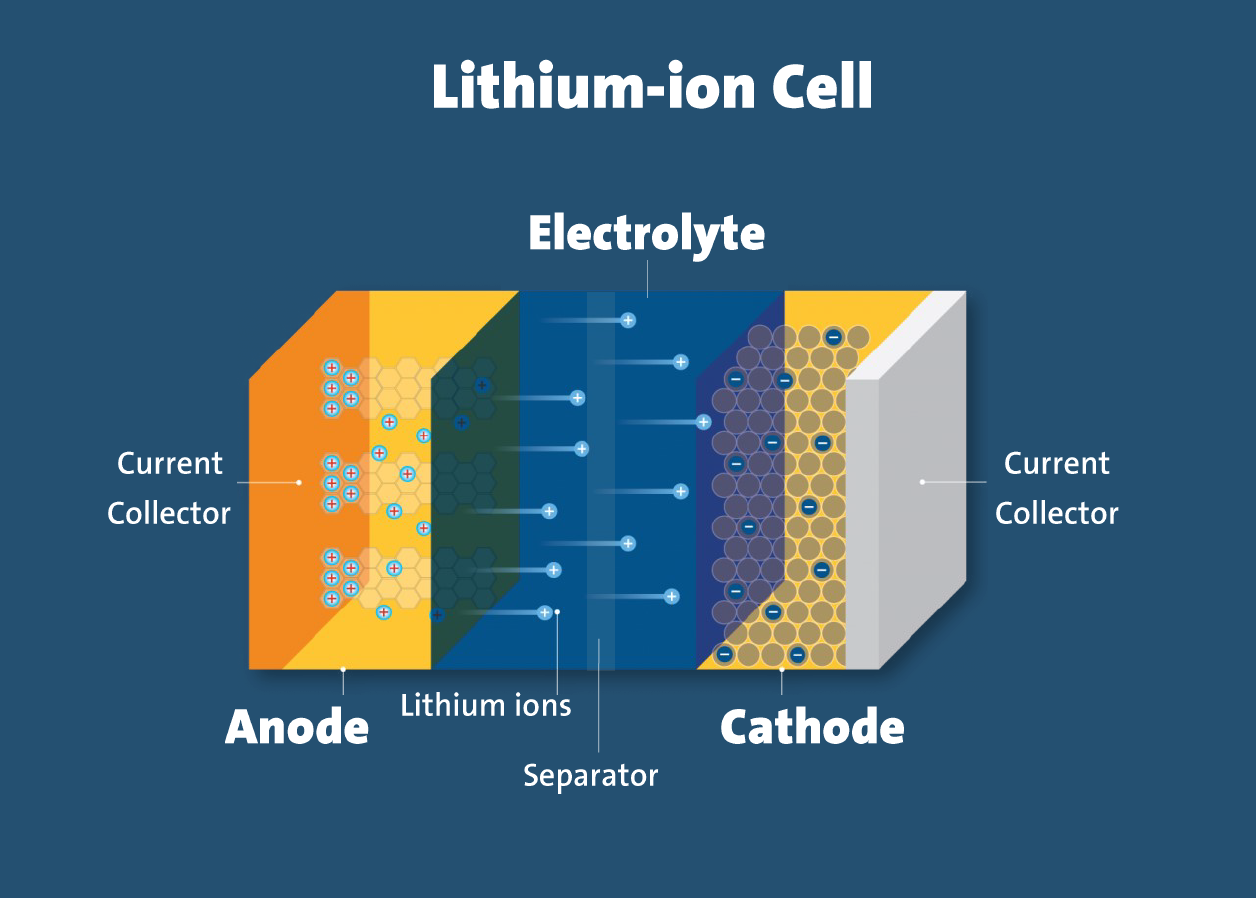
- Electrodes: The positively and negatively charged ends of a cell. Attached to the current collectors
- Anode: The negative electrode
- Cathode: The positive electrode
- Electrolyte: A liquid or gel that conducts electricity
- Current collectors: Conductive foils at each electrode of the battery that are connected to the terminals of the cell. The cell terminals transmit the electric current between the battery, the device and the energy source that powers the battery
- Separator: A porous polymeric film that separates the electrodes while enabling the exchange of lithium ions from one side to the other
How does a lithium-ion cell work?
In a lithium-ion battery, lithium ions (Li+) move between the cathode and anode internally. Electrons move in the opposite direction in the external circuit. This migration is the reason the battery powers the device—because it creates the electrical current.
While the battery is discharging, the anode releases lithium ions to the cathode, generating a flow of electrons that helps to power the relevant device.
When the battery is charging, the opposite occurs: lithium ions are released by the cathode and received by the anode.
PUBLISHED